Learning Outcomes:
i. Describe the general electron configuration of d-block elements.
ii. Explain the filling of d orbitals in d-block elements.
iii. Analyze the factors that influence the variability in oxidation states of d-block elements.
iv. Connect the electronic configuration of d-block elements to their magnetic properties.
Introduction:
D-block elements, also known as transition metals, occupy the middle portion of the periodic table, encompassing Groups 3 to 12. These elements exhibit unique electronic structures and chemical properties due to the involvement of d orbitals in their electron configuration. In this lesson, we will explore the electronic structures of d-block elements, unraveling the arrangement of electrons and their implications for their chemical behavior.
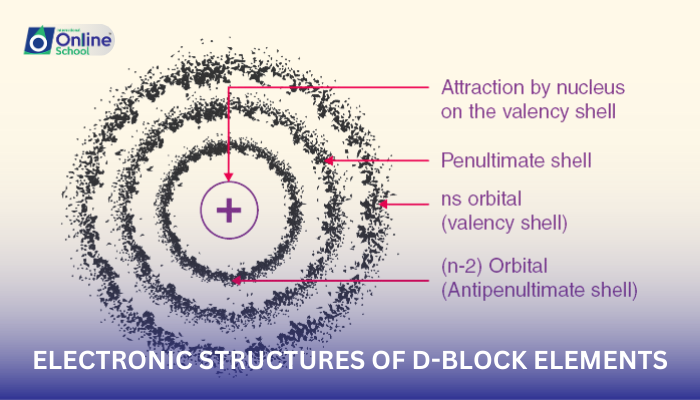
i. General Electron Configuration
D-block elements follow a general electron configuration of ns2np2nd1-10ns1, where n represents the outermost principal energy level. The distinguishing feature of their configuration is the presence of electrons in the d orbitals of the penultimate energy level (n-1).
The filling of d orbitals follows the Aufbau principle, filling orbitals with progressively higher energy. However, exceptions to this rule arise due to energy level overlaps and pairing energies. The number of electrons in the d orbitals varies from one to ten, contributing to the diverse chemical behavior of d-block elements.
ii. Variability in Oxidation States
D-block elements exhibit a remarkable variability in oxidation states, meaning they can lose a varying number of electrons to form compounds. This variability is attributed to the involvement of d orbitals in bonding and the ability of electrons to be promoted to higher energy levels.
For instance, iron can exhibit oxidation states of +2 and +3, forming compounds such as iron(II) oxide (FeO) and iron(III) oxide (Fe2O3). This variability is due to the availability of d electrons in iron that can be lost or shared in bonding.
iii. Magnetic Properties
The electronic configuration of d-block elements also influences their magnetic properties. Unpaired electrons in d orbitals contribute to the magnetic moment of an atom or ion. Elements with unpaired d electrons exhibit paramagnetism, while those with all d orbitals paired are diamagnetic.
For example, iron(II) ions with four unpaired d electrons are paramagnetic, while copper(I) ions with all d orbitals paired are diamagnetic. This understanding of magnetic properties is crucial in various fields, including materials science and catalysis.
The electronic structures of d-block elements provide a fundamental understanding of their chemical properties and behavior. The filling of d orbitals, the variability in oxidation states, and the connection to magnetic properties are all aspects that shape the unique chemistry of these elements. By understanding the electronic structures of d-block elements, we can predict their chemical reactivity, bonding patterns, and applications in various fields.